Clinical Vignette 30
A middle-aged woman presented with fever and lethargy, and was diagnosed with acute myeloid leukaemia (AML) after thorough work-up. She underwent standard induction chemotherapy (IA 3+7) and received fluconazole prophylaxis in view of cost concerns. She developed neutropenic fever 3 days after completion of chemotherapy, and was prescribed IV imipenem after an extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae was isolated from blood cultures. However, her fever failed to settle after a further 4 days despite blood cultures having cleared. A CT thorax at this point showed a nodule abutting the pleura in the right upper lobe of her lung (figure).

CT thorax slice of the woman with persistent febrile neutropenia, showing a right upper lobe nodule.
The serum galactomannan was repeatedly negative, and she was treated with voriconazole empirically. However, her fever persisted for another week until her counts recovered, after which it spontaneously lysed. A repeat bone marrow aspiration showed that her disease was in remission, but a repeat CT thorax performed 2 weeks after her counts had recovered showed that the nodule was still present, and had in fact increased in size by approximately 15%.
She would have to undergo consolidation chemotherapy in order to improve her chances for achieving long-term remission from her AML.
Question: How would you deal with the problem of her lung nodule, particularly in light of her need for further chemotherapy?
[Updated 2nd May 2015]
Despite the negative serum galactomannan result and apparent “failure of response” to voriconazole, invasive fungal disease (IFD) remains the most likely diagnosis given the patient’s clinical profile. The serum galactomannan test is specific to aspergillosis and a small number of other IFDs (viz. histoplasmosis), and is moreover not particularly sensitive. Prof John Wingard and his colleagues had published an excellent clinical approach to the issue of pulmonary nodules in patients with haematological diseases here. Basically, because of the change in immune status (recovery of white cell counts), existing IFD nodules can increase in size even if antifungal therapy had been microbiologically successful, and a repeat CT thorax at a period of two weeks post-cell count recovery is probably too short (in terms of timing of the scan from count recovery) to determine if antifungal therapy had failed in such a patient.
For this patient, there were two possible approaches:
- A more conservative approach with continuation of voriconazole with repeat of CT thorax another 2-4 weeks later (and continuing voriconazole therapy through the subsequent consolidative phase of her AML therapy).
- A more aggressive approach with video-assisted thoracoscopic surgery (VATS) to remove the solitary pulmonary nodule. This has the advantages of confirming the diagnosis of IFD and at the same time treating it definitively (via surgical removal), but the trade-off includes potential risks from anaesthesia and surgery, as well as delay of the consolidative AML chemotherapy until a reasonable period post-operation in order to ensure wound healing.
We elected for the second approach, and fortunately the operation went well without complications, while the delay in chemotherapy did not affect her significantly (she remained in remission subsequently for a further few years). The histology was consistent with IFD (image below), and fungal cultures from the intra-operative specimen were negative.
However, we were able to identify the fungus via ITS sequencing of fungal DNA from the tissue – this has been well described in an earlier post by the SGH microbiology blog. The invading fungus in this case turned out to be Rhinocladiella spp., a phaeohyphomycete (i.e. black mold) that has rarely been involved in human infection – specifically in leukaemic patients or those requiring stem cell transplantation who become profoundly neutropenic. this fungus is generally considered to be susceptible to azoles such as voriconazole and posaconazole, although there is little experience given the rarity of infections by this particular fungus.
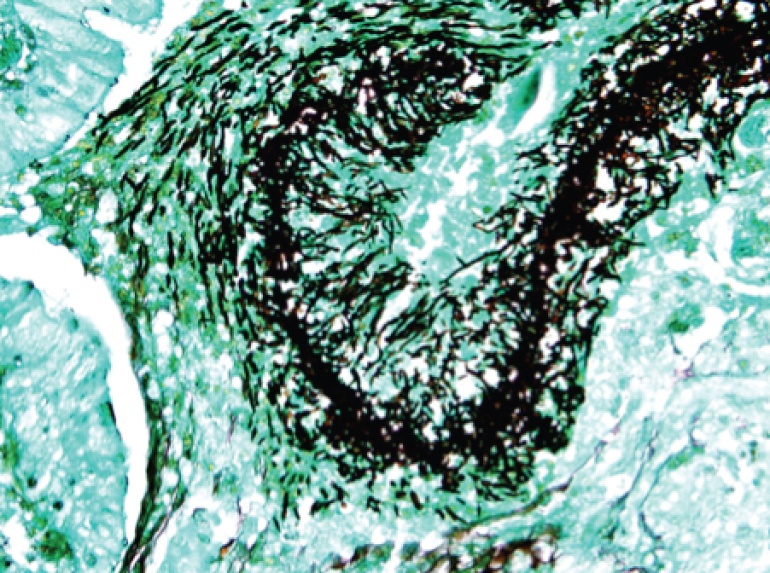
VATS biopsy
LikeLiked by 1 person