
Type VI Secretion System and Antimicrobial Resistance in Acinetobacter spp.
The rather boring term “type VI secretion system” (it is the 6th bacterial secretion system discovered) refers to a remarkable molecular contractile “sting” that can be assembled and disassembled, and is found in multiple Gram-negative bacteria. Its primary purpose – as the term suggests – is to secrete proteins into another cell. It is deployed against other competing bacteria (nature on a microscopic level, “red in tooth and claw”), although various Gram-negative bacteria – such as Vibrio cholerae where it was originally described or Franciscella tularensis – have adapted it to target eukaryotic cells, contributing to their pathogenicity.
The figure below is a nice schematic of a typical type VI secretion system, which I have copied and pasted from Francesca Cianfanelli and colleagues’ excellent review in Trends in Microbiology published 2 years ago. The article is unfortunately behind a pay wall, but here is another open access article that reviews all the bacterial secretion systems, from type I to VI.

Type VI secretion system. The bacteria assembles the construct within its cytoplasm, and “fires” it by contracting the sheath and extruding the inner tube out through the cell wall. PAAR and VgrV form the puncturing “sting” that perforates the target cell, after which the “cargo” effector proteins are pumped into the target cell’s cytoplasm. ClpV (green) then disassembles the sheath.
The type VI secretion system typically delivers a “payload” that comprises a variety of antibacterial (and anti-eukaryotic in specific bacteria) toxins that target multiple sites within the competitor cell, including those that target the cell membrane, cell wall and the chromosome. Below is another figure obtained from an excellent review in Cellular Microbiology, which is well worth reading. How do bacteria avoid being destroyed by the type VI secretion systems of their own species? They build specific “immunity proteins” against the payloads of their own kind.

Screen shot of a figure from Juliana Diniz and colleagues’ review in Cellular Microbiology, 2015.
What does this have to do with Acinetobacter spp. and antimicrobial resistance? for some time now, it is known that Acinetobacter spp. have a rather “plastic genome”, with frequent recombinations and insertions into its chromosome. Acinetobacter baumannii has also developed resistance to antibiotics rapidly, and is arguably the most drug-resistant bacteria found in hospitals worldwide. This has largely been through the process of horizontal gene transfer, although why it happens so easily for Acinetobacter spp. is not well known. But in a pair of articles published late last year, scientists from the University of California, U.S.A. and University of Basel, Switzerland show one possible major mechanism.
Under laboratory settings, Robert Cooper and colleagues from the University of California demonstrated that “predatory” Acinetobacter baylyi was able to acquire antimicrobial resistance genes from Escherichia coli cells that had been destroyed via the type VI secretion system of the former. The following cartoon, screen-captured from the manuscript, shows a conceptual view of the process.

Screen capture from the eLife paper on A. baylyi predatory cell obtaining genetic material from the E. coli prey cell.
As the predatory A. baylyi lysed E. coli prey cells, the genetic material of the lysed cell could be “scavenged” by A. baylyi, and utilised. There were a number of interesting experiments that tested the hypothesis in the manuscript, which were quite thoroughly thought out. I have picked a figure panel from the manuscript demonstrating the rise in kanamycin-resistant A. baylyi even as kanamycin-resistant E. coli population fell over time.
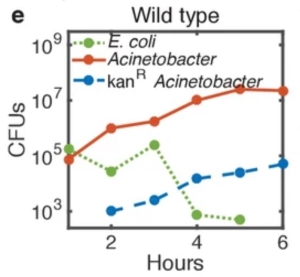
Peter Ringel and team, in a separate publication in Cell Reports shortly after Cooper et al, essentially confirmed the results, and also described the molecular process and activation of the A. baylyi type VI secretion system in much greater detail.
It is not clear if this model of antibiotic resistance gene acquisition operates in all Acinetobacter spp. (A. baylyi is non-pathogenic, while we are more concerned with its more virulent “relations” in the A. baumannii-calcoaceticus complex), but the same type VI secretion system is also present in the latter, with similar effector proteins.